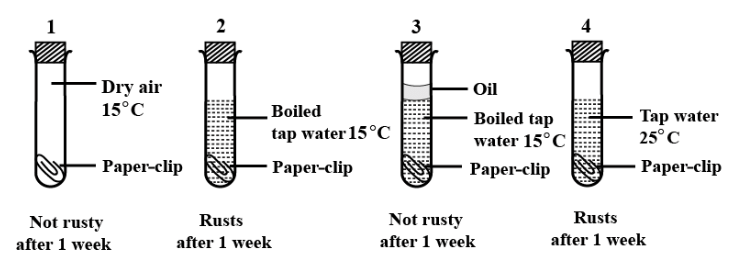
When iron starts to rust, what’s actually happening is a chemical change—something entirely new gets created in the process: iron oxide. For this to occur, both oxygen and moisture (either water or even just water vapor in the air) have to be present.
Rusting doesn’t happen all at once; it’s a gradual process that keeps going over time, steadily damaging iron objects. Eventually, this persistent rust can ruin the iron, leaving it weak and often unusable.
Is rusting of iron a chemical change?
A chemical change is what happens when one or more new substances come into existence as a result of a reaction. Take rusting, for instance if you leave iron out where it can meet air and moisture, you’ll soon spot a reddish coating forming on its surface.
That’s rust, which is actually iron oxide—a completely new substance created when iron reacts with oxygen from the air. Not only does the surface color change, but the material itself becomes something else, which is why rusting is considered a classic example of a chemical change.
When we talk about chemical changes, we often mention rusting, but it’s just one example. The broader term for rusting and related processes is “corrosion.”
There are other everyday words that point to chemical changes too think of burning, rotting, exploding, or fermenting. These all describe situations where the original material is transformed into something new.
Now, if you’re trying to figure out what makes a substance unique, its chemical properties are a good place to look. For example, iron’s chemical property is its ability to combine with oxygen and form iron oxide rust, in other words.
But here’s the catch: unlike physical properties (like color or melting point, which you can check without changing the substance), you can only observe a chemical property while the substance is actually changing into something else. That’s what makes chemical properties so interesting—and so useful for telling substances apart.
A chemical change often referred to as a chemical reaction is what happens when substances transform into entirely new substances. Let’s use zinc as an example. Zinc, with the symbol Zn, appears as a silvery gray metal, and you can grind it down into a fine powder if you like.
Now, imagine you have some powdered sulfur as well. Sulfur (symbol S) stands out with its bright yellow color. If you simply mix powdered zinc and sulfur together at room temperature, you won’t see anything dramatic it’s just a basic blend, and neither substance changes on a chemical level. They’ll sit together in the container, side by side, but nothing new is formed.
But here’s where things get interesting: add heat to this mixture. When you supply enough energy in the form of heat, something changes. The zinc and sulfur don’t just sit together anymore they react. The result is a brand new substance called zinc sulfide, or ZnS.
Think of it this way: you start with two elements zinc (A) and sulfur (B). When you heat them, they combine in a chemical reaction and create a compound, zinc sulfide (C).
Chemists like to represent reactions like this with a chemical equation. In plain English, you could describe the process as:
zinc + sulfur → zinc sulfide
But most of the time, we use chemical symbols and formulas because it’s quicker and more precise. So, the reaction is written as:
Zn + S → ZnS
In these equations, everything on the left side of the arrow the zinc and sulfur are called the reactants. Reactants are simply the substances you start with before the reaction takes place.
On the right side of the arrow, you have the product in this case, zinc sulfide which is what you end up with once the reaction is complete. So, to sum it up: zinc and sulfur react when heated, and together, they form a completely new substance, zinc sulfide.

Recognizing Chemical Reactions
How do you know when a chemical reaction is happening? There are actually a few signs you can look out for, though they don’t guarantee a reaction every time. Some of the classic giveaways include:
- A change in color: If you notice the substances shifting in color as they interact, that’s often a clue.
- Gas production: Sometimes, you’ll see bubbles or fizzing as a reaction takes place—this is a sign that a gas is being released.
- Formation of a solid (precipitate): Occasionally, you’ll spot a solid suddenly appearing in the solution, which chemists call a precipitate.
- Energy changes: You might even catch a flash of light or another visible transfer of energy as the reaction happens.
Let’s look at some real examples. Take zinc reacting with hydrochloric acid: as soon as you mix them, you’ll see bubbles racing to the surface. That bubbling? It’s hydrogen gas being produced, and it’s a pretty strong hint that a chemical change is underway.
Or consider what happens if you add a colorless solution of lead (II) nitrate to a colorless solution of potassium iodide. Almost immediately, a bright yellow solid appears—that’s your precipitate (specifically, lead iodide). The appearance of this solid is a classic sign that a chemical reaction has taken place.
The chemical equation for this reaction looks like this:
Pb(NO₃)₂(aq) + 2 KI(aq) → PbI₂(s) + 2 KNO₃(aq)
So, whether you spot bubbles, see a sudden color shift, or notice a new solid forming, those are all signals that something new is being made right before your eyes.
What is Physical Changes?
Physical properties describe aspects of a substance that can be observed or measured without altering its chemical identity. These include attributes like shape, size, state (solid, liquid, gas), and colour. One key point is that physical properties can change when the substance undergoes a physical transformation.
Take ice, for example. When it melts into water, what you’re witnessing is a shift from a solid to a liquid state. If you reverse the process and cool the water, it turns back into ice.
This kind of change doesn’t create any new substance; you’re simply toggling between different forms of the same material. The process is reversible, which is a hallmark of many physical changes.
Think about what actually happens as ice melts: its rigid structure breaks down, it starts to flow, but at the molecular level, it’s still just H₂O. The identity remains unchanged. That’s the essence of a physical change some properties (like state or appearance) may shift, but the underlying substance stays the same.
Physical changes themselves fall into two categories: reversible and irreversible. Changes of state, like melting, freezing, condensation, and vaporization, can all be undone by adjusting temperature or pressure they’re reversible.
Dissolving is another example. If you stir salt into water, you can later recover the salt simply by evaporating the water; the original substances can be separated without any chemical reaction.
However, not every physical change is so easily undone. For instance, grinding wood into sawdust is irreversible; there’s no practical way to reconstruct the original piece of wood from its shavings. The same logic applies when grass is cut or a rock is crushed these changes can’t be reversed.
Similarly, once a tree is chopped into firewood, those pieces can’t be reassembled into the original tree. These examples illustrate the concept of irreversible physical changes, where the form is altered beyond recovery, but the chemical nature of the material stays intact.
FAQs
What is the process of rusting of iron?
Rust is a type of corrosion that occurs on metal surfaces when iron reacts with oxygen and water. The chemical reaction that causes rust is called oxidation. The oxidation process eats away at the metal and creates visible rust on the metal’s surface.
Why rusting of iron is a serious problem?
When iron is exposed to oxygen and water, it corrodes, resulting in the formation of an iron oxide known as rust. Corrosion of iron is a severe problem since it reduces the beneficial characteristics of iron, causing it to lose strength and eventually become unsuitable for use.
What is the chemical reaction of rust?
The chemical formula for rust is Fe2O3 and is commonly known as ferric oxide or iron oxide. The final product is a series of chemical reactions simplified below as- The rusting of the iron formula is simply 4Fe + 3O2 + 6H2O → 4Fe(OH)3. The rusting process requires both the elements of oxygen and water.
What happens when iron gets rusted?
The oxygen atoms bond with iron atoms, resulting in the formation of iron oxides. This weakens the bonds between the iron atoms in the object/structure. The reaction of the rusting of iron involves an increase in the oxidation state of iron, accompanied by a loss of electrons.
How to make iron rust faster?
How to do it quickly
1. White vinegar is the chemical which causes most of the rusting effect.
2. Lemon juice will provide you with quite a surprising effect and appearance.
3. Muriatic acid and peroxide is quite a popular way to quickly rust metal.
How long does it take for iron to start rusting?
Compared to the corrosion of other metals, iron rusts relatively quickly, especially if it is exposed to water and oxygen. In fact, when iron is exposed to water and oxygen, it can begin to rust within a few hours. Iron will also rust quickly if it’s exposed to high temperatures.