Structure of Graphite
Graphite is a fascinating example of a giant covalent structure. If you take a close look, you’ll find that each carbon atom connects with three others, forming strong covalent bonds.
These atoms organize themselves into layers, each one shaped like a flat sheet of hexagons, almost like chicken wire.
What’s particularly interesting is that while the bonds within each layer are quite strong, the layers themselves are only loosely held together by weak forces. This arrangement explains why graphite feels so slippery and can be used as a lubricant or pencil “lead.”
Trying to sketch out graphite’s three-dimensional structure can be tricky it’s not the kind of thing that’s easy to represent on paper. But if you imagine a stack of honeycomb-shaped sheets, spaced just the right distance apart, you’re on the right track.
Carbon, as an element, can take on several forms these are called allotropes and graphite and diamond are the two best-known. The difference between them comes down to the way the carbon atoms bond.
In diamond, each carbon forms four strong, directional (sp3) bonds, arranging itself into a rigid tetrahedral network. That’s why diamonds are so hard. In graphite, however, each carbon uses three sp² hybrid orbitals to bond with its neighbors, creating flat planes where each atom is joined to three others at 120-degree angles.
These planes themselves are often called graphene layers. Zooming in, you’d see each carbon atom sitting at the corner of a hexagon, all spaced by a bond length of 0.142 nanometers. The gap between one layer and the next is about 0.335 nanometers close enough to stack neatly, but not so close as to stick together tightly.
Within a single layer, the covalent bonds hold firm. But with only three out of four possible bonds occupied for each carbon atom, there’s a leftover electron. This loose electron is what gives graphite its ability to conduct electricity along the planes a property that makes graphite unique among nonmetals.

In graphite, the fourth electron from each carbon atom isn’t tied down; instead, it can move freely across the plane of the layers. This mobility is what gives graphite its impressive electrical conductivity within the layers.
When it comes to the connections between these layers, things are quite different: only weak van der Waals forces hold them together. That’s why graphite can be so easily split into thin sheets or why the layers slide past one another with minimal effort.
As a result, if you try to send electricity through graphite perpendicular to the layers, you’ll find it’s roughly a thousand times less conductive in that direction.
Now, if you’ve ever tried to draw a side-on diagram of graphite, you’ve probably noticed a problem. It’s almost impossible to represent both the scale of the atoms within a layer and the spacing between layers accurately in the same picture either the atoms get squished together or the layers end up ridiculously far apart.
So, to give you a sense of scale: the gap between two layers is about 2.5 times greater than the distance between atoms within a single layer.
It’s also worth pointing out that these graphite layers aren’t just a handful of atoms stuck together; they actually stretch across vast numbers of atoms far more than any simple illustration can show.
You might be wondering, though: since carbon typically forms four bonds (thanks to its four unpaired electrons), why does each carbon in graphite seem to bond with only three neighboring carbons in these diagrams? That’s a fair observation.
The diagrams you often see are a bit of a shortcut they focus on showing the overall arrangement of atoms and don’t capture every detail of the bonding.
The Bonding in Graphite
When you look at a single layer of carbon atoms like what you find in graphene each carbon connects with three neighboring atoms, using up three of its electrons in the process. But that’s not the whole story. After forming those bonds, every carbon still has one electron left over that isn’t locked into place.
Instead of staying put, these leftover electrons spread themselves out over the entire carbon sheet. They don’t belong to just one atom or even a specific pair. Think of them as drifting freely across the surface, not pinned down by any particular bond.
The real takeaway here is how much freedom these delocalized electrons have. Since they’re not attached to any single atom, they can move wherever they want within the layer. This gives the whole structure its distinct electronic properties something that sets it apart from many other materials.
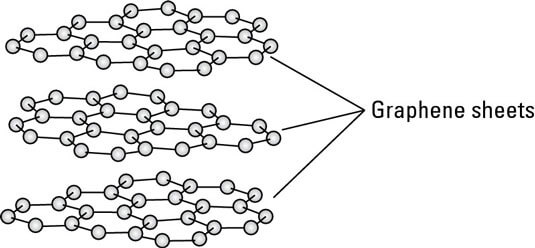
Interestingly, the delocalized electrons found in one layer of graphite don’t actually make direct contact with those in the layers above or below.
Within a single layer, however, the atoms are bonded tightly together by strong covalent bonds. In fact, these bonds are even stronger than those in diamond, thanks to the added stabilization from the delocalized electrons.
But what about the forces that keep the layers stacked together? In graphite, this is where van der Waals dispersion forces come into play. As electrons move freely within each sheet, they can create sizable, temporary dipoles.
These, in turn, induce opposite dipoles in neighboring layers almost like a ripple effect spreading through the entire graphite structure.
When it comes to physical properties, graphite is pretty remarkable. It boasts a melting point that’s just as high as diamond’s. If you wanted to melt graphite, you couldn’t just separate the layers; you’d need to break apart those sturdy covalent bonds across the entire structure.
Graphite is also known for being soft and slippery. That’s why it’s so useful in things like pencils or as a dry lubricant for locks and hinges.
You can actually picture graphite like a stack of playing cards: each card (or sheet) is strong on its own, but the stack can slide apart quite easily. When you write with a pencil, layers of graphite are sheared off and left behind on the paper.
Another interesting aspect is that graphite is less dense than diamond. This comes down to the gaps between the layers, which leave a fair bit of unused space.
As for solubility, graphite doesn’t dissolve in water or most organic solvents just like diamond. The attraction between the carbon atoms in graphite and the molecules of a solvent isn’t nearly strong enough to break the network of covalent bonds.
Finally, graphite conducts electricity. The freely moving delocalized electrons travel along the layers, so when you hook up a piece of graphite to a circuit, electrons can exit from one end while new electrons enter from the other.
FAQs
What best describes the structure of graphite?
As previously touched upon, graphite has a planar, layered structure; each layer being made up of carbon atoms linked together in a hexagonal lattice. These links, or covalent bonds as they are more technically known, are extremely strong, and the carbon atoms are separated by only 0.142 nanometres.
What is the formula for graphite structure?
Graphite is an allotrope of carbon and therefore has a chemical formula of C. In other words, graphite is made only of carbon, therefore its chemical formula is the same as the chemical symbol of carbon (C). The chemical symbol for carbon can be found on the periodic table.
Which has graphite like structure?
Boron Nitride (BN) resembles the structure of graphite. It is known as the inorganic graphite and it has a structure similar to that of graphite.
What type of structure is graphite?
Graphite has a giant covalent structure in which: each carbon atom is joined to three other carbon atoms by covalent bonds. the carbon atoms form layers with a hexagonal arrangement of atoms.
What are the structural characteristics of graphite?
Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Graphite thus crystallizes in the hexagonal system, in contrast to diamond, another form of carbon, that crystallizes in the octahedral or tetrahedral system.